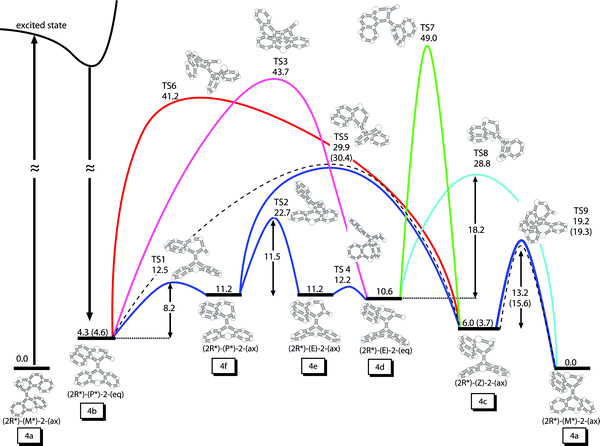
Fig. 5: Fig. 5 RI-MP2/TZVP energies at the B3LYP/6-31G* geometries of the six equilibrium conformers of 2 and the corresponding transition states between them. Energies are given in kcal/mol. Values in parenthesis are taken from ref. 29. Dashed lines refer to the pathway suggested in ref. 29. The labels 4a–4f correspond to the isomers of Fig. 4.
DOI
10.1039/c0cp00324g
Abstract
Chiral overcrowded alkenes are capable of unidirectional rotation via a series of cis-trans photochemical and helix-inversion thermal steps. Using a pseudo-random conformational search we have located different ground state minima belonging to the potential energy surface of two different overcrowded alkenes that function as molecular rotors. The transition states connecting the minima allow identifying different reaction pathways which are possible in the thermal helix-inversion steps. The mechanisms found for the two studied molecular rotors are different and provide a valuable insight into the conformational dynamics of the rotary cycle. While in one case the thermal step occurs via a single transition state, in the other, several intermediates are accessible. The associated energy barriers are in agreement with the experimental values, supporting the proposed mechanisms.